Graft-versus-Host Disease (GvHD)
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for adult patients with high-risk acute leukemia or severe hematopoietic failure syndromes. Overall survival is within the range of 25 to 85 % for leukemia patients depending on primary disease, stage, conditioning regimens and risk groups. However, allo-HSCT is still associated with major complications, such as severe acute or later chronic graft-versus-host disease (GvHD) and infections.
Acute GvHD (aGvHD)
Depending on the type of transplantation, patient age, immunosuppressive prophylaxis, and underlying hematologic disease, 35-85% of transplanted patients develop aGvHD. First line therapy of aGvHD consists of steroids resulting in a response rate of about 70% for patients with aGvHD grade I or II without significant increase of mortality. In contrast, patients developing aGvHD grades III or IV have a mortality risk of about 80-90% due to aGvHD-induced organ dysfunction or concomitant infections. Differential diagnosis of aGvHD from treatment related toxicities can be difficult and is mainly made according to clinical symptoms and biopsies. Thus, a method is urgently needed to diagnose early onset of aGvHD and to identify patients at risk of developing severe GvHD in an observer-independent, unbiased fashion.
CE–MS has been applied to identify biomarkers for early detection of aGvHD in patients undergoing allo-HSCT since 2003. Mosaiques employed these biomarkers to generate an aGvHD-specific classifier, named aGvHD_MS17 that allow distinction of patients with severe aGvHD from those who never develop aGvHD and patients with low or moderate aGvHD.
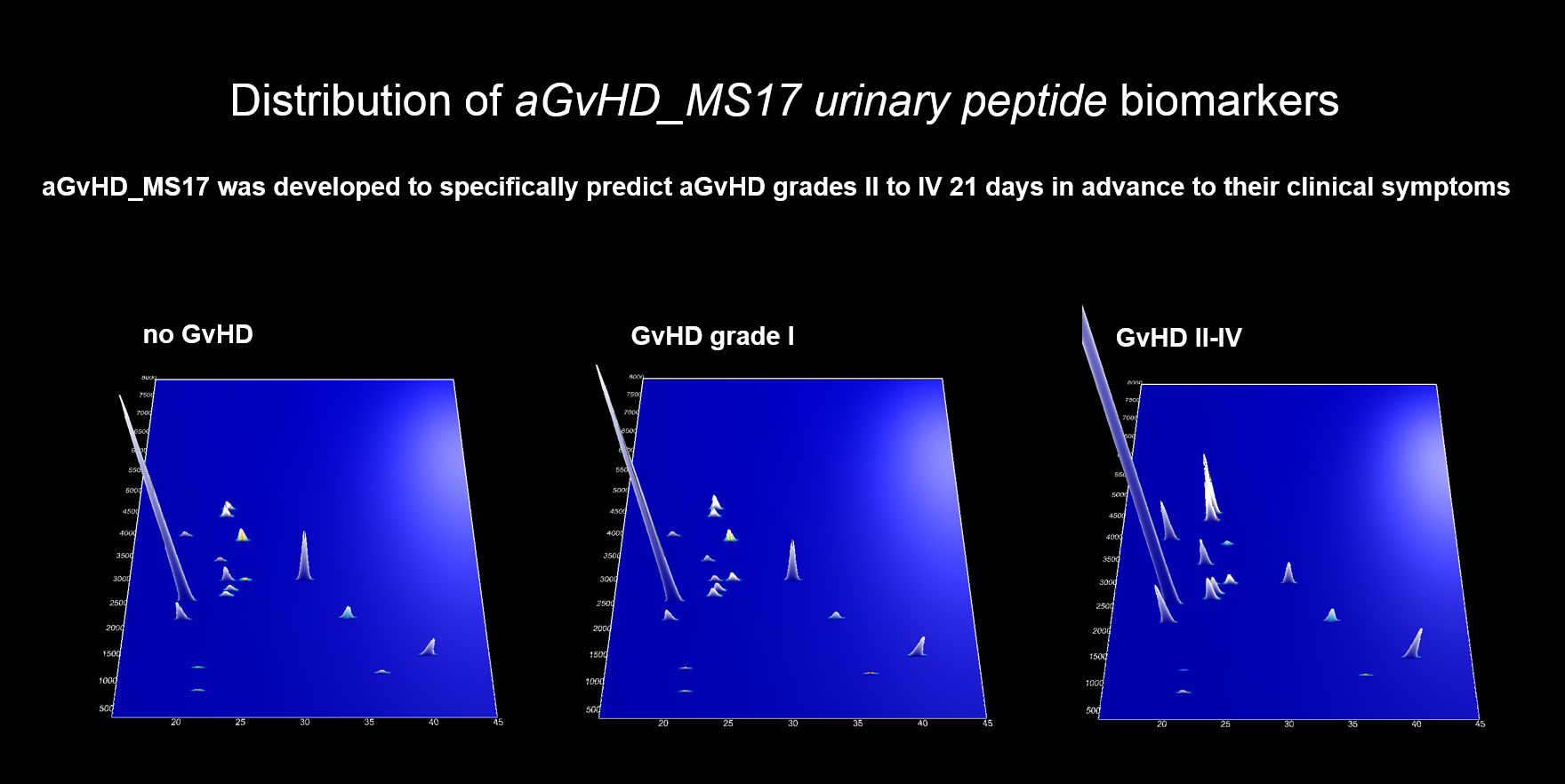
Recently the predictive value of aGvHD_MS17 was evaluated in two large prospective studies. The first study was conducted with the objective to detect severe aGvHD 21 days in advance to its clinical signs and results were published 2014 in the scientific journal Leukemia. This study included 463 leukemia patients after stem cell transplantation between 2005 and 2010 enrolled in five German transplant centres. It represents the largest study using proteomics in clinical patient assessment. In this study, it was found that aGvHD_MS17 enabled the diagnosis of severe aGvHD with a sensitivity and specificity of 72.6 and 78.6 % for its own and 82.4 and 77.3 % when combined with relevant demographic and medical patient variables, respectively.
In 2008, a second multicenter, randomized, placebo-controlled, double blind clinical trial was started to test the applicability of aGvHD_MS17 to the clinic as a tool to predict severe aGvHD and to initiate pre-emptive steroid therapy (Trial registration: ISRCTN03911524). Eleven German transplant-centres contributed 267 patients to this trial and 92 were randomized according to the positivity of aGvHD-MS17 to receive either the steroid prednisolone or placebo.
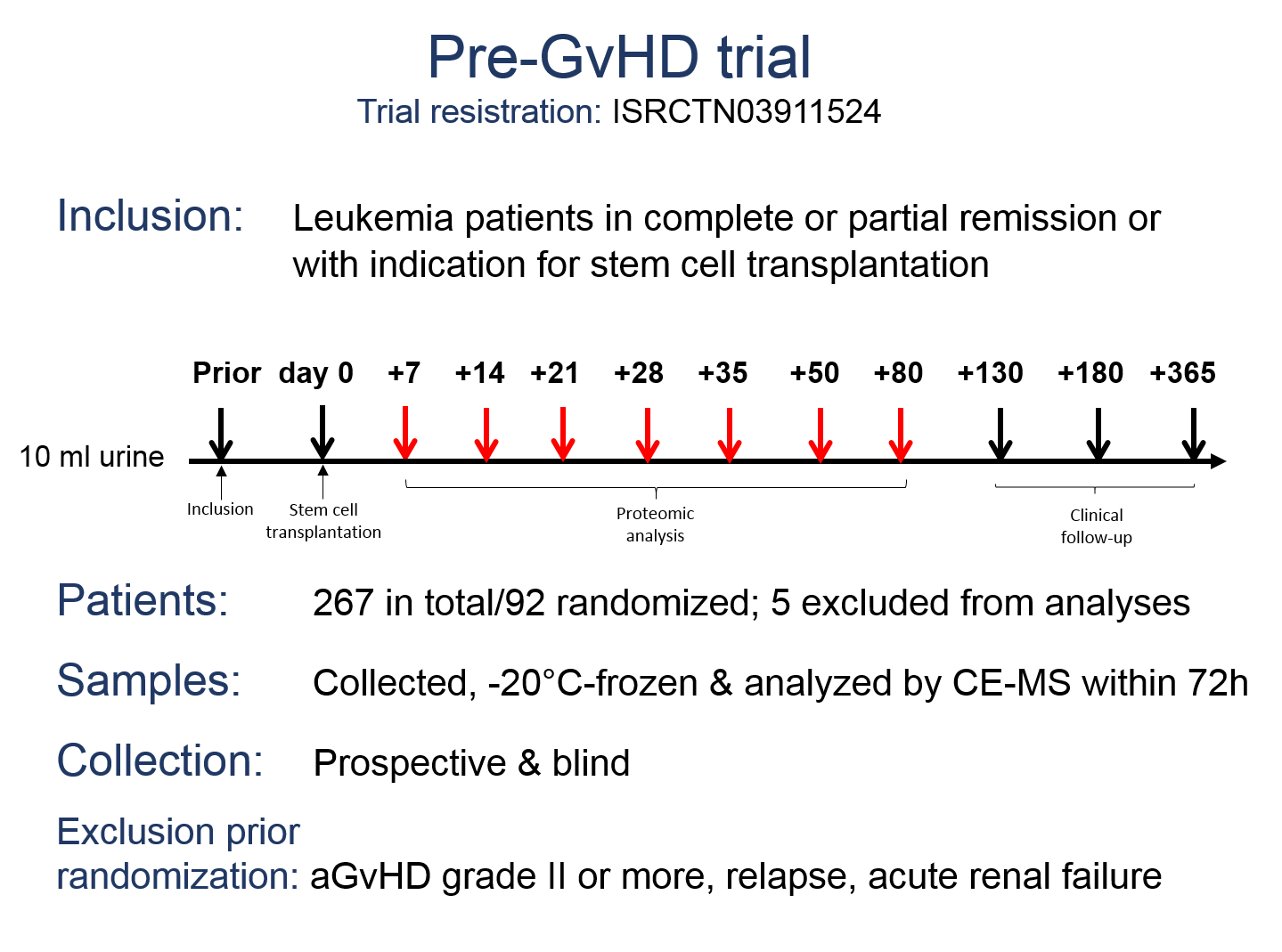
As reported at the ASH meeting in December 2017, prospective and blinded evaluation of aGvHD_MS17 in the Pre-GvHD trial revealed that the first analysis time point (day +7; range: 2-17) was most accurate in the prediction of aGvHD grade II or more with a sensitivity of 87% and a specificity of 81%. A high sensitivity and specificity was also observed on analyses time points day +14 and day +21, but the pattern lost its predictive value by day +28. Patients with samples positive for aGvHD_MS17 in the early time points had a 21-fold higher risk to develop aGvHD grade II or more, when compared to patients with negative samples. Confounding factors for accurate diagnosis of aGvHD grade II or more at the later time points are conditioning according to RIC-protocols and early death after stem cell transplantation.
Both large prospective multicentre studies thus clearly demonstrate the high predictive power, clinical usefulness and applicability of this novel diagnostic tool in post-transplantation surveillance of leukemia patients after hematopoietic stem cell transplantation.
REFERENCES
- Weissinger EM et al. Ann Hematol 2006; 85(4): 205-211.
- Weissinger EM et al. Blood 2007; 109(12): 5511-5519.
- Weissinger EM et al. Leukemia 2014; 28(4): 842-52.
- Weissinger EM et al. Leukemia. 2021; 35(6): 1763-1772.
Chronic GvHD (cGvHD)
Chronic GvHD (cGvHD), the protracted form of GvHD, occurs in 35-50 % of stem cell transplanted patients, hampers their quality of life and leads to increased morbidity and mortality by inflammation- and fibrosis-mediated organ damage even years after transplantation.
Currently, diagnosis of cGvHD is based on clinical features and histological examinations of affected tissues and thus diagnosis can only be made at a time point where clinical manifestations of the disease have already evolved.
A proteomic peptide marker pattern was established by Mosaiques for early diagnosis of cGvHD, to differentiate it from acute GvHD, and to predict its onset and severity. The cGvHD_MS14 classifier was prospectively evaluated over a time period of 9 years on 422 patients at four German transplant centres. Prospective and blinded evaluation revealed the correct classification of patients developing cGvHD with a sensitivity of 92.5% and a specificity of 75.3%. Acute GvHD prior to day 100 is not recognized by cGvHD-MS14 and its classification factors are correlated to the severity of cGvHD according to the Seattle limited and extensive cGvHD nomenclature.
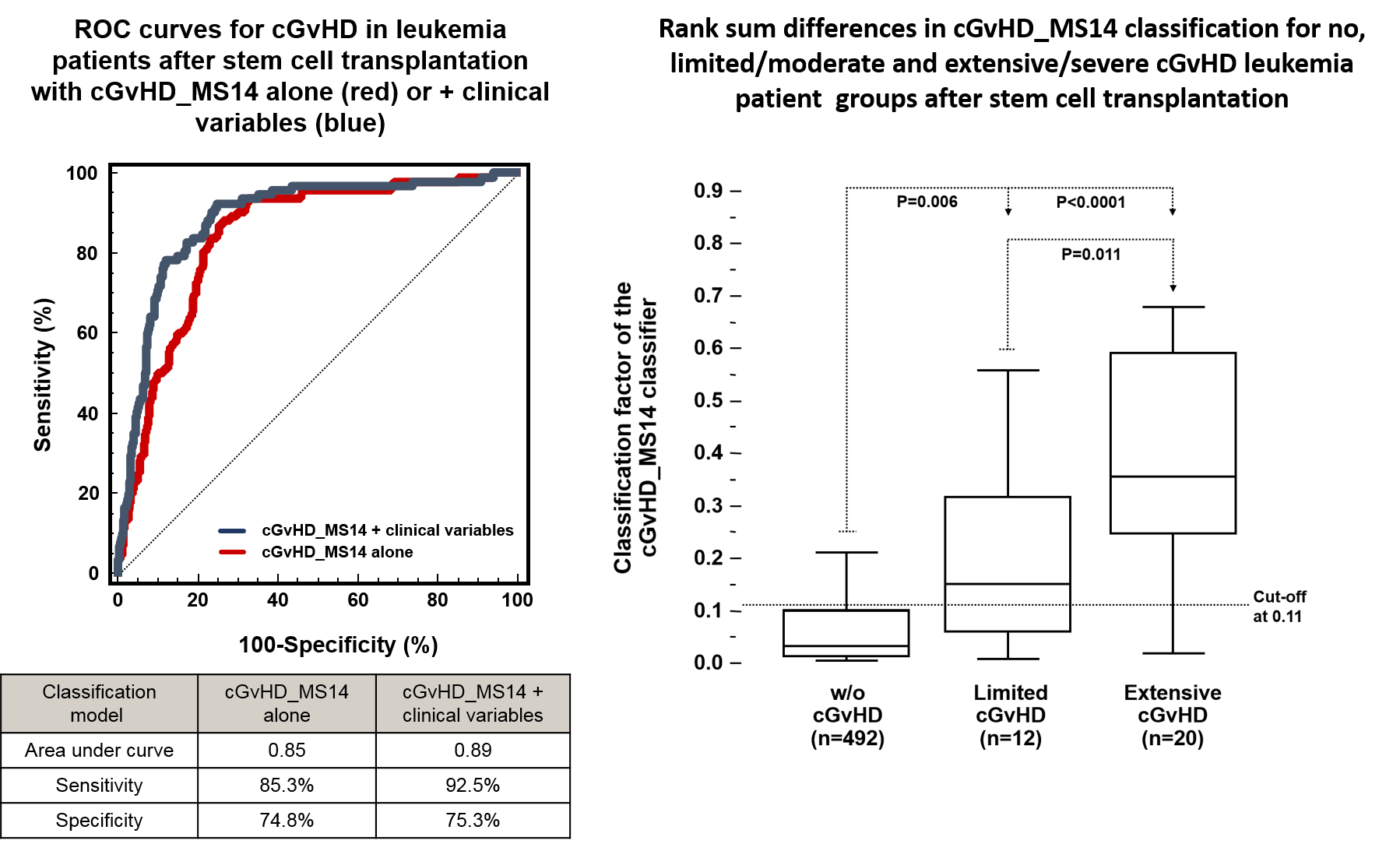
Urinary proteomic monitoring by cGvHD_MS14 introduces the first unbiased, investigator-independent diagnosis strategy of moderate and severe cGvHD and shall increase safety and feasibility of clinical trials for cGvHD.
REFERENCES
- Weissinger EM et al. Leukemia 2017; 31(3): 654-662.
- Weissinger EM et al. Expert Rev Proteomics 2020; 17(3): 201-206.



