Pediatric Nephrology
In pediatric nephrology, physicians are frequently confronted with congenital anomalies of the kidney and urinary tract (CACUT). CAKUT constitute approximately 20-30% of all anomalies identified in the prenatal period. Defects can be bilateral or unilateral, and different defects often coexist (Figure 1). Because CAKUT plays a causative role in 30-50% of cases of ESRD in children, it is important to diagnose these anomalies and initiate therapy to minimize renal damage, preventing or delaying the onset of ESRD, and providing supportive care to avoid complications of ESRD. CAKUT manifest as structural abnormalities of the kidney, including obstructive uropathy, renal dysplasia, and urinary tract malformations. CAKUT displays an extensive spectrum of prenatal and postnatal outcomes alternating from death in utero to normal postnatal renal function..
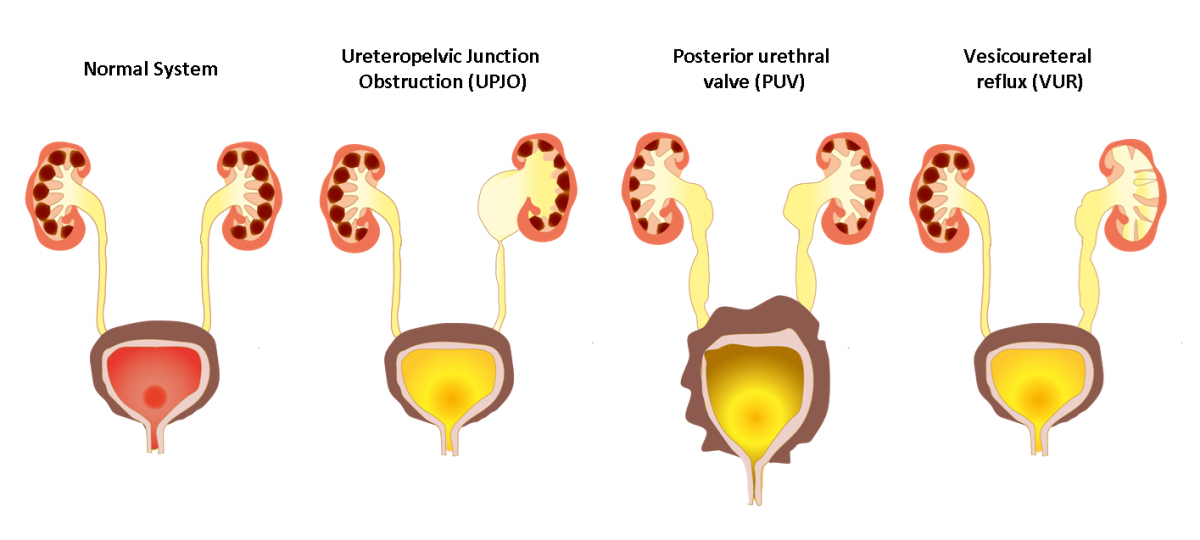
Figure 1. Different types of congenital anomalies of the kidney and urinary tract (CACUT).
Ureteropelvic junction obstruction (UPJO) is a frequent cause of congenital obstructive nephropathy and is characterized by a stenosis between the ureter and the kidney, inducing accumulation of urine in renal pelvis and calyces, called hydronephrosis. In severe cases this condition is treated surgically. However, in the milder UPJO cases (often) invasive surveillance is necessary during the first years of life to determine whether surgery is necessary. Urinary proteome analysis was employed to determine the presence of urinary markers that could predict the progression of UPJO at an early stage. The combination of different urinary peptides in a UPJO-classifier predicted progression of UPJO with the need of surgical intervention several months in advance. Furthermore, this classifier was validated in an independent study with pediatric patients. In children <1 year old, the model for UPJO showed 83% sensitivity and 92% specificity. On the other hand, in older patients, the analysis yielded a sensitivity and specificity of 20% and 66%, respectively. This suggests that classifiers should be validated within their context of use, in this case detection of severe UPJO before the age of 1 year. In addition, we used urinary proteome analysis to suggest that early surgery in UPJO might be beneficial compared to classical conservative clinical surveillance of the disease. At 5-year follow-up urinary proteomes were similar between patients with early surgical correction of UPJO and age matched controls. In contrast, urinary proteomes differed significantly between conservatively followed patients and controls. Analyses of the proteome differences suggested ongoing renal or ureteral remodeling in the conservatively followed patients that was not clinically visible.
Posterior urethral valves (PUV), the prototypic bilateral CAKUT is an obstructing membrane in the posterior male urethra. We analysed the urine of PUV patients with CE-MS and identified fetal urinary peptides associated with fetuses with PUV displaying early ESRD. These peptides were combined in a PUV-classifier, which allowed correct prediction of postnatal renal function with 88% sensitivity and 95% specificity, in an independent blinded cohort of PUV patients.
High-grade vesicoureteral reflux (VUR) is described by an abnormal condition of flow of the urine from the bladder to the kidney during micturition. This condition is a risk factor for impaired renal function, renal scarring and arterial hypertension, although is not a frequent condition in children. Current diagnosis requires an invasive and highly uncomfortable method - voiding cystourethrography (VCUG). Therefore, we investigated children urine with the use of CE-MS analysis and a VUR-classifier was established in patients with primary VUR grade IV or V, distinguishing these from patients without VUR. This VUR-classifier was independently validated in a blinded cohort of patients with VUR grade IV or V and patients without VUR with a sensitivity of 88% and a specificity of 79%.
To improve prenatal prediction of postnatal kidney survival in CAKUT, we conducted a prospective multicenter proteome analysis of amniotic fluid spanning 140 evaluable fetuses with CAKUT. We identified a signature of 98 endogenous amniotic fluid peptides, mainly composed of fragments from extracellular matrix proteins and from the actin binding protein thymosin-β4. The peptide signature predicted postnatal kidney outcome with an area under the curve of 0.96 in the holdout validation set of patients with CAKUT with definite endpoint data. Additionally, this peptide signature was validated in a geographically independent sub-cohort of 12 patients (area under the curve 1.00) and displayed high specificity in non-CAKUT pregnancies (82 and 94% in 22 healthy fetuses and in 47 fetuses with congenital cytomegalovirus infection respectively). Thus, recognition of the 98-peptide signature in amniotic fluid during diagnostic workup of prenatally detected fetuses with CAKUT can provide a long-sought evidence base for accurate management of the CAKUT disorder.
Other pedriatic kidney diseases
Furthermore, we generated diagnostic tests for the detection of Renal Cysts and Diabetes (RCAD) syndrome in children and for chronic antibody-mediated rejection (cABMR) of pediatric kidney transplants. For more details see: Ricci P et al., 2019 and Kanzelmeyer NK et al., 2019.
REFERNCES
Decramer S et al. Nat Med 2006:12(4):398-400.
Drube J et al. Pediatr Nephrol 2010:25(9):1673-8.
Caubet C et al. Pediatr Nephrol. 2010; 25(1):27-35.
Bandin F et al. J Urol 2012:187(3):1006-11.
Drube J et al. Pediatrics 2012;129(2):e356-e363.
Klein J et al. Sci Transl Med 2013;5(198):198ra106.
Klein J et al. Expert Rev Proteomics 2014;11(1):75-89.
Schanstra JP et al. Pediatr Nephrol. 2015; 30(5):713-25.
Magalhães P et al. Expert Rev Proteomics. 2016; 13(12):1121-1129.
Kanzelmeyer NK et al. Transpl Int. 2019; 32(1):28-37.
Ricci P et al. Sci Rep. 2019; 9(1):2225.
Klein J et al. Kidney Int. 2021; 99(3):737-749.



