Kidney allograft rejection
Acute rejection is an important factor that determines long-term function and survival of the renal allograft. Approximately 15-30% of the transplanted patients suffer from one or multiple acute rejections, which occur mainly in the first year of transplantation.
Timely detection and sufficient anti-rejection therapy of acute rejection episodes is important to conserve the allograft function. However, detection of acute rejection at an early stage is challenging. Regular monitoring for increases in serum creatinine or decrease in creatinine clearance, with subsequent indicated biopsy, implies that the rejection is detected in an advanced stage where the graft is already impaired by the rejection process.
Extending a first proof-of concept study on 26 patients with acute rejection, Mosaiques established a peptide marker pattern in urine for detection of T cell-mediated allograft rejection at the early subclinical level. The peptide pattern was validated in a cohort of 25 patients with and 43 patients without rejection resulting in a sensitivity of 91% and a specificity of 76%. In further validation on a blinded test set of 17 patients, the marker pattern enabled correct classification of 6 out of 7 patients with acute rejection and 10 out of 11 patients negative for rejection.
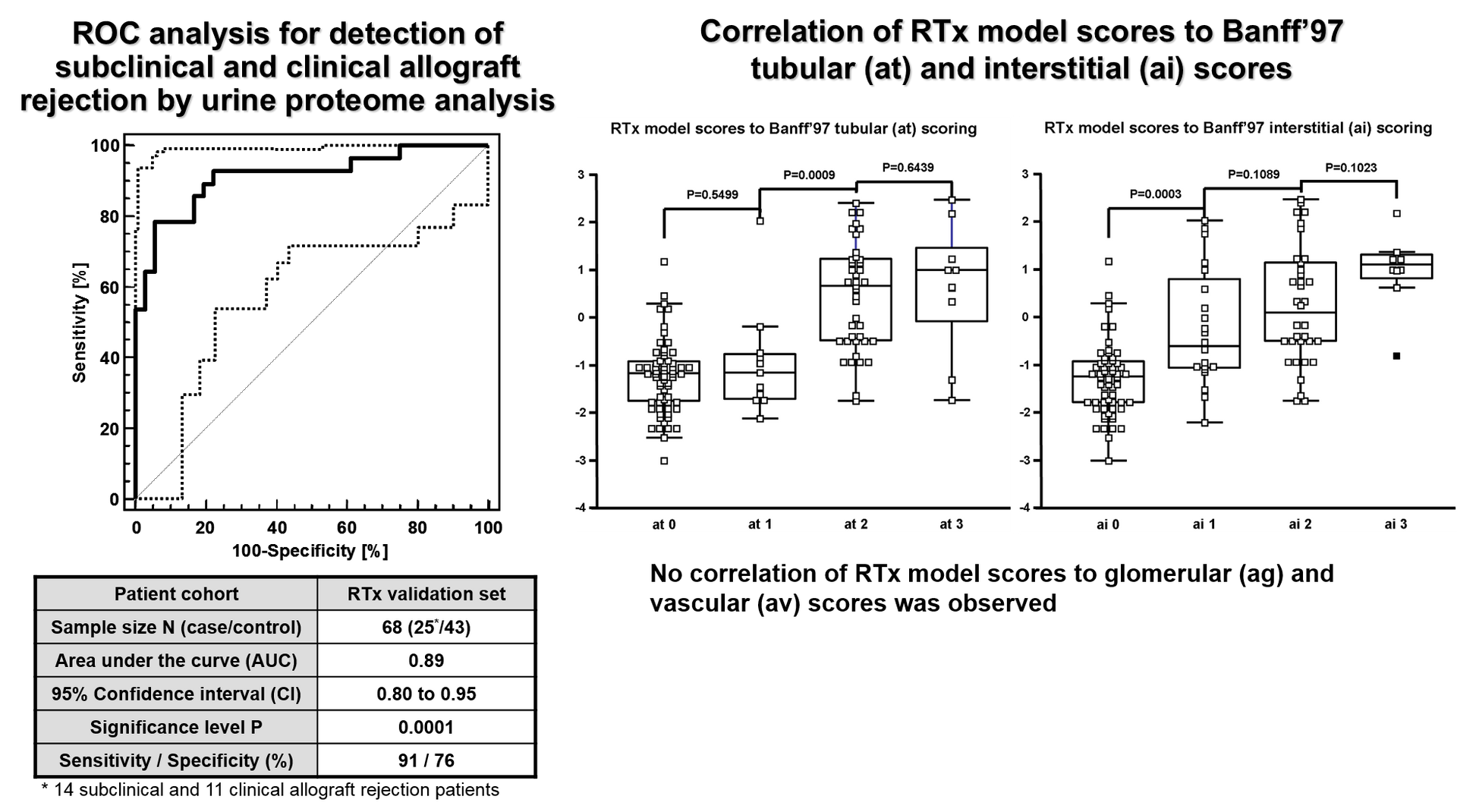
On the basis of this case-control study, Mosaiques initiated together with the Department of Nephrology of the Medical School Hannover a prospective clinical trial in 2011 to examine the diagnostic accuracy of the mass spectrometry test in urine for the diagnosis of acute rejections using the biopsy as gold standard (Trial registration: NCT01315067). In this study, which is described from its conception and design by Zapf et al. [4], more than 600 patients within the first year of transplantation who are scheduled for a biopsy due to unexplained impairment of the allograft were included. Mass spectrometry analysis of patient samples ended in November 2016 and the studies’ results are currently under investigation by the Institute for Biostatistics of the Medical School Hannover.
Moreover, Mosaiques is a scientific partner of the European R&D project “BIOMARGIN” (BIOMArkers of Renal Graft INjuries in kidney allograft recipients), aiming at discovering and validating robust non-invasive biomarkers for the follow up of renal grafts (for details see the link: http://www.biomargin.eu).
REFERENCES:
- Gwinner W. World J Urol 2007; 25: 445-455.
- Wittke S et al. Am J Transplant 2005; 5: 2479-88.
- Metzger J et al. Proteomics Clin Appl. 2011; 5: 322-33.
- Zapf A et al. BMC Nephrol. 2015; 16: 153.
- Gwinner W et al. World J Transplant. 2016; 6: 28-41.
- Kanzelmeyer NK et al. Transpl Int. 2019; 32(1): 28-37.
