Clinical Proteomics
In healthy individuals, a distinct peptide pattern exists. Peptides in body fluids (urine, serum, bile, cerebrospinal fluid etc.) control the fate of cells, and consequently of organs and organisms. Hence, a thorough display of the proteins present in body fluids at any given physiological situation should give insights into the regulatory mechanisms of most diseases and support exact and unbiased diagnosis. Any changes in these peptides are indicative or, in some cases, even the cause for most of the common diseases. Our approach is to utilise the peptide pattern found in body fluids to obtain information about the stage of disease and the general health of the patient. In case of an emerging or existing disease proteins are missing, emerge in different formations, are modified or additional proteins appear. Consequently, recovery of the normal pattern indicates a successful therapy, which predestines our approach for control of therapy and monitoring of drugs in preclinical and clinical research.
The combination of capillary electrophoresis (CE) coupled online to a time-of-flight mass spectrometer (TOF-MS) is the method of choice to accomplish the needs for clinical proteomics. Our CE-MS approach is fast, reproducible and suitable for high throughput screening. During the last years we have analysed samples of over 100,000 patients in collaboration with academic partners and pharmaceutical companies. Our intensive endeavours yielded in the description of many biomarkers and the development of a unique relational database in the context of genitourinary, cardiovascular and oncological diseases.
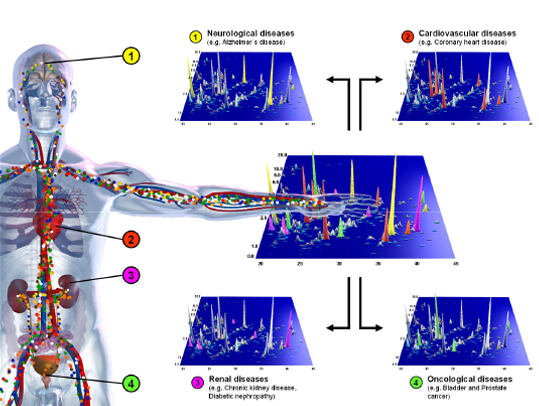
Figure 1: Changes in a distinct and defined pattern of peptides in body fluids will allow enormous improvements in diagnosis and therapy for many wide-spread diseases, for example neurological diseases (1), cardiovascular (2), and renal (3) diseases, bladder cancer (4) and prostate cancer (4).
Our CE-MS technology offers several advantages: speed (less than 60 min per run), high resolution and reproducibility. The dataset from individual analyses can be compiled to generate a typical proteome pattern that can be based on >100 individual analyses. From our point of view urine is, besides cerebrospinal fluid, bile and serum, the body fluid of choice for diagnosis of diseases and monitoring of therapy outcome. Urine is easily accessible, stable (over 10 years, if stored at -20°C), available in large quantities and obtained in a non invasive, painless and riskless procedure. Urinary peptides display the state of health of the kidney, bladder, prostate, biliary system and vascular architecture. The combination of peptides and proteins form biomarker pattern, which are more capable to display the health status of an organ or organism with improved significance. Furthermore, disease specific biomarker patterns tolerate instability and inconsistency of individual peptides/biomarkers. Our proprietary software solutions enable automated and standardized data interpretation. The very high diagnostic accuracy of our method is reflected by sensitivities and specificities between 85% and 95%. Further characterisation of defined biomarkers is possible via CE-MS/MS sequencing to enable their use in additional applications (e.g. immunological assays).
The implementation of our CE-MS technology is possible from the very beginning of preclinical development (animal models) until clinical trials (phase I-IV). We are highly experienced in the efficient and time-saving assessment of newly developed as well as already existing pharmaceutical substances. We believe that our approach in clinical proteomics enables the accurate clinical characterisation of patients and controls (high fidelity phenotyping). CE-MS profiling will accelerate drug development by ascertainment of statistically significant data from a small number of patients (defined cohorts) plus an enhancement in the determination of pharmaceutical drug safety.

Figure 2: Costs and time of drug development can be significantly reduced by mosaiques´ solutions for clinical proteomics. Additionally, drug safety is improved by the early determination of side-effects.
For instance, our technology, enables the differentiation of progressor/non-progressor of diabetic nephropathy five years in advance or the early development of heart failure. In addition, a single measurement can be explored for different parameters and questionings, e.g. side-effects of a pharmaceutical drug for the treatment of diabetic nephropathy on the cardiovascular system. Examining the efficacy and side-effects of newly developed pharmaceutical substances (monitoring) by our approach will help the pharmaceutical industry to minimize the duration and the size of cohorts of (pre)clinical studies. Obtained peptide patterns help to select an appropriate therapy, which is general benefit for patients. Peptide patterns are suitable for precise description of an organ and its alterations, and allow displaying dose related therapeutic effects of administered drugs.
