Cholangiocarcinoma
Cholangiocarcinoma (CC) is a rare but highly aggressive adenocarcinoma of the bile ducts accounting for 3% of all gastrointestinal cancers and 15% of all primary liver cancers worldwide. CC is diagnosed in 2.000-3.000 new cases on average per year in the United States, which is translated to an annual incidence of 1-2 per 100,000 and year worldwide. The incidence for CC is still rising per year by approx. 3%.
Due to poor response to chemotherapy, early surgery is the only curative treatment option. However, surgery is associated with 5-10 % mortality and even with margin-free resection 5-year survival rates only reach 20-40 %. In 50-95% of cases, CC is detected in patients at a stage too late for resection. In this case, the prognosis is poor with the median survival being 5 months. Due to this poor outcome, CC accounts for 10-20 % of deaths by hepatobiliary malignancies which in turn constitute 13% of the annual cancer-related deaths worldwide (7.8 million deaths in 2008 according to the WHO worldwide statistics).
The detection of CC remains a diagnostic challenge particularly in patients with primary sclerosing cholangitis (PSC), a chronic cholestatic liver disease of unknown etiology characterized by progressive inflammation and fibrosis of the bile ducts. Patients with PSC are known to develop CC in approximately 30% of cases during the first 10 years of the disease. Currently, CC is detected using endoscopic imaging and histology obtained during endoscopy but has at best 60% sensitivity.
Two case-control studies performed by Mosaiques together with the gastroenterology department of the Medical School Hannover, using bile and urine as sample matrix, the former for local activity and the later for systemic signs of CC progression, indicate that proteomic analysis is a feasible method to detect CC in patients with PSC or other benign biliary disorders by peptide multi-marker models with classification accuracies above 80%. Urine proteome analysis in this respect enables non-invasive monitoring of patients at risk for CC.
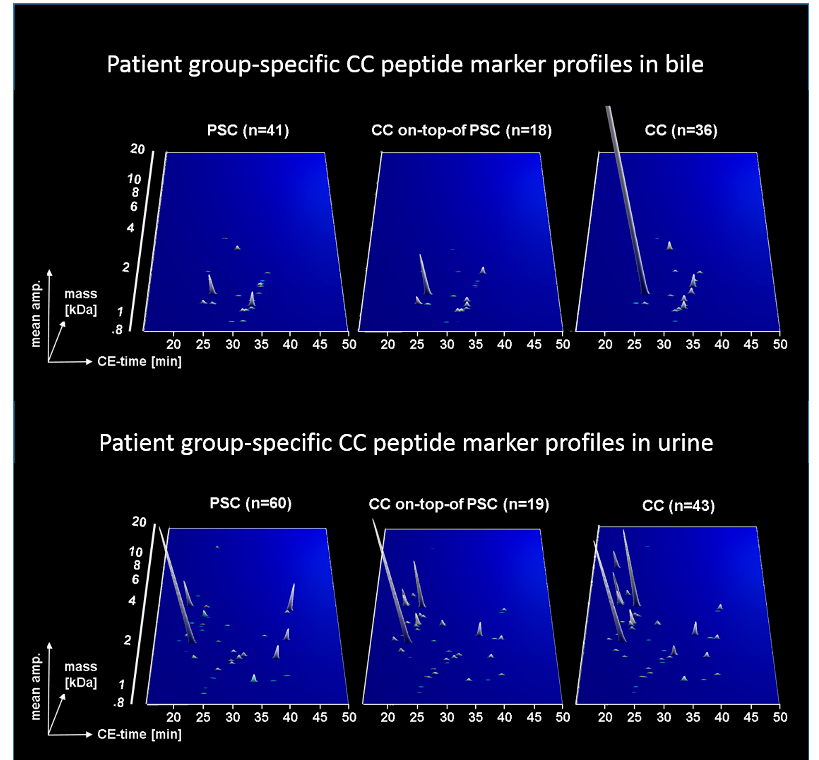
Later it was found that a logistic regression model based on both proteomic tests further improved the accuracy of CC diagnosis at the date of endoscopy.

In a prospective evaluation on subsequently collected patients with a six months clinical follow-up, the logistic regression model led to an accuracy for CC detection of 92 %. The BPA/UPA test is thus most applicable for patients with biliary strictures of unknown origin and PSC patients under surveillance referred to endoscopy due to progressive cholestasis.
REFERENCES:
- Lankisch TO et al. Hepatology 2011; 53: 875-84.
- Metzger J et al. Gut 2013; 62: 122-30.
- Voigtländer T et al. United European Gastroenterol J 2017; 5: 668-76.
- Voigtländer T et al. J Biomed Sci. 2020; 27(1): 13.
