Bladder cancer
Bladder cancer is the most common malignancy of the urinary tract. It is caused by abnormal cell growth leading to a mass of tissue (tumour) in bladder lining. Based on the global statistical estimates (GLOBOCAN analysis [4]), the estimated number of newly diagnosed cases in 2012 were 429,000 patients and 165,000 bladder cancer related deaths. Limited treatment options as well as the invasive nature of cystoscopy that is used for life-long monitoring of the patients, decreases patient‘s life expectance and quality of life and make bladder cancer the most costly cancer to treat.
Bladder cancer stages
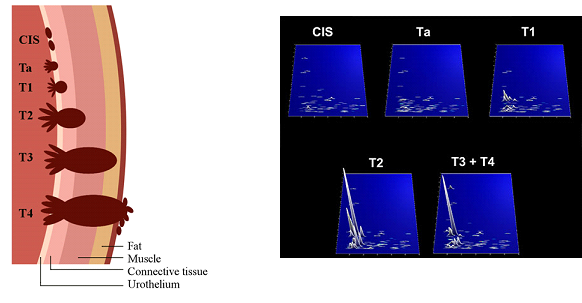
Depending on the level of infiltration of the cancerous cells through layers of bladder wall, bladder cancer is characterised as:
a) Non-muscle invasive (NMIBC; Stages Ta, T1, CIS) and
b) Muscle invasive (MIBC: Stages T2, T3, T4).
Risk Factors
- Cigarette smoking – considered as a main risk factor for bladder cancer. Cigarette smoking is associated with half of bladder cancer cases.
- Working environment - Exposure at workplace to carcinogenic chemicals such as aromatic hydrocarbons, aromatic amines and chlorinated hydrocarbons, especially in the various parts of industry (i.e. production of dyes, rubber, textile, leather or chemicals).
- Age, Gender – Risk of developing bladder cancer increase with age and bladder cancer is more frequently detected in men than in women, with men to women incidence ratio at around 3:1.
- Medical history of the patients including presence of urinary tract infections, schistosomiasis, previous treatment (radiotherapy, chemotherapy).
Symptoms of Bladder Cancer
Hematuria (presence of blood in urine) is a first sign of early stage bladder cancer. As tumour advances, other symptoms may appear including increase frequency and painful urination or unexpected need to urinate. In the case of the infiltration of the tumour into muscle layer, pelvic and bone pain, loss of weight, inability to urinate or swelling of feet may occur.
Early diagnosis and monitoring is of paramount importance
At initial diagnosis the vast majority of bladder cancer patients (70-80%) present with early stage non-muscle invasive bladder cancer (NMIBC), 20-30% present with advanced stages with muscle invasion (MIBC) and approximately 5% exhibiting clinically evident distant metastases. With the treatment options being limited, basically radical cystectomy is the main option for treating muscle invasive disease, the outcome is very poor and more than 50% of the patients with MIBC disease, deceasing within 5 years. At the same time, NMIBC is related with very high recurrence rates of up to 78%. Therefore, early detection is of paramount importance to improve disease outcome.
Up to now, the diagnosis of bladder cancer is performed using cystoscopy. The accuracy in detecting BC is not always sufficient, while the procedure is highly invasive. Based on the high numbers of cystoscopies that are carried out during the follow-up period, bladder cancer remains one of the most expensive and most uncomfortable cancer type worldwide. Unnecessary and unpleasant cystoscopies and the associated risks can be prevented using the test that are developed based on the urinary proteomic analysis based on capillary electrophoresis coupled to mass spectrometry (BCU -Bladder Check-Up- test).
Scientific Background
In the context of bladder cancer, Capillary Electrophoresis coupled to Mass-Spectrometry technology (CE-MS) was initially employed by Mosaiques Diagnostics GmbH in 2006. The study was designed to support the discovery of biomarkers that could detect bladder cancer in the healthy population. A CE-MS based biomarker panel that was developed and validated, exhibited good discriminatory capabilities for detecting primary BC [12]. A second biomarker panel was later developed to discriminate between non-muscle and muscle invasive disease, as described in an article published in Clinical Cancer Research [7].
Detection of Recurrence and guiding Monitoring
Bladder cancer is characterized by high relapse/recurrence rates. Therefore, frequent patient monitoring is required, generating a prevailing need for non-invasive biomarkers for timely diagnosis of BC, as recently outlined in several articles [2, 3].
To address the need for monitoring bladder cancer, a large multi-center study was conducted to discover and validate urinary biomarker panels for detecting primary and recurrent BC. The study that was recently published in Clinical Cancer Research [1], included 5 clinical centers and in total 1357 urinary profiles from patients with bladder cancer or those suspicious for the disease. Two biomarker panels were developed for primary and recurrent urothelial bladder cancer, based on 116 and 106 peptide biomarkers, respectively. In the independent validation phase, the 116 peptide biomarker panel for primary UBC presented sensitivity at 91% with specificity of 68% (AUC=0.87). The 106 peptide biomarker panel for monitoring recurrence presented sensitivity and specificity values of 88% and 51% (AUC=0.75), respectively. In reference to the peptide biomarker model for detection of recurrence, combination with cytology increased the AUC to 0.87.
The significance of the results was outlined in Nature Reviews Urology Journal (Thoma 2016, Nat Rev Urol), reporting that the established biomarker panels enable the detection of primary and recurrent bladder cancer with high sensitivity and specificity. This study resulted in the introduction of the Bladder Check-Up (BCU) test and the Bladder Status Managment (BSM) test of protexam.
The above study was a result of a collaborative effort within a European Consortium, Building upon a long and fruitful collaboration, the Consortium is devoted to translational research and implementation of protein biomarkers for the management of urological cancers.
BCU test/ BSM test
The BCU test/ BSM test is a novel gentle method to diagnose bladder cancer using highly sophisticated technologies. By screening with the BCU test/ BSM test, the disease can be timely detected at early stages where more treatment options are possible.
Benefits of screening by protexam
The five-year survival average rate of bladder cancer is 77%. Early and timely detection followed by therapy, increases the chances of recovery to 96.2% at early stage.
protexam screening is also suitable for aftercare
The relapse rate in bladder cancer is particularly high up to 78%. Therefore a follow-up session for the early re-emerging tumors in 1st and 2nd year after treatment should be initiated every 3 months. This decrease in invasive diagnostic procedures clearly reduces patients‘ discomfort during the follow-up and remarkably increases the patients‘ quality of life.
The BCU test/ BSM test and the application of the mosaiques technology in bladder cancer have been described in several publications [1-3, 6, 8-12, 14-17, 19].
REFERENCES:
- Frantzi M, et al. Development and Validation of Urine-based Peptide Biomarker Panels for Detecting Bladder Cancer in a Multi-center Study. Clin Cancer Res. 2016 Aug 15;22(16):4077-86.
- Mengual L, et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clin Cancer Res. 2016, 22(16):4077-86.
- Frantzi M, et al. Developing proteomic biomarkers for bladder cancer: towards clinical application. Nat Rev Urol. 2015, 12(6):317-30.
- Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015, 136(5):E359-86.
- Witjes, J.A. et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014, 65(4):778-92.
- Latosinska A, et al. Clinical applications of capillary electrophoresis coupled to mass spectrometry in biomarker discovery: Focus on Bladder Cancer. Proteomics Clin Appl. 2013, 7(11-12):779-93.
- Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013.
Eur Urol 2013, 64(4):639-53. - Siwy J, et al. Clinical proteomics: Current techniques and potential applications in the elderly. Maturitas. 2011 Mar;68(3):233-44
- Schiffer E. Urinary proteomics: ready for prime time in urological cancer diagnostics? Personalized Medicine 2011, 8 (1): 81-94
- Schiffer E. The 2nd annual oncology biomarkers conference. Biomark Med. 2009 Apr;3(2):203-9.
- Schiffer E, et al. Prediction of Muscle-invasive Bladder Cancer Using Urinary Proteomics. Clin Cancer Res. 2009, 15(15):4935-43
- Schiffer E, et al. Proteomics in gerontology - current applications and future aspects. Gerontology. 2009, 55(2):123-37.
- van Rhijn, B.W. et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol 2009, 56(3):430-42.
- Schiffer E, et al. Challenges of using mass spectrometry as a bladder cancer biomarker discovery platform. World Journal of Urology 2008, 26(1): 67-74.
- Wittke S, et al. Kapillarelektrophorese gekoppelte Massenspektrometrie zur Proteomanalyse: Eine innovative diagnostische Methode bei Prostata- und Blasenkrebs. Der Urologe 2007, 46(7): 733-739.
- Mischak H. Hochauflösende Proteomanalyse aus Urin in der nicht invasiven Diagnostik von Tumoren und chronischen Erkrankungen. prevention and anti aging 2006, 2(4): 426-435.
- Theodorescu D, Wittke S, Ross MM, Walden M, Conaway M, Just I, Mischak H, Frierson HFet al. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006, 7(3): 230-240.
- Sylvester, R.J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006, 49(3):466-5; discussion 475-7.
- Kolch W, et al. The molecular make-up of a tumour: proteomics in cancer research. Clin Sci (Lond). 2005, 108(5): 369-383.
