FDA issues letter of support for urinary peptide-based biomarker to detect early stage of CKD
June 2016
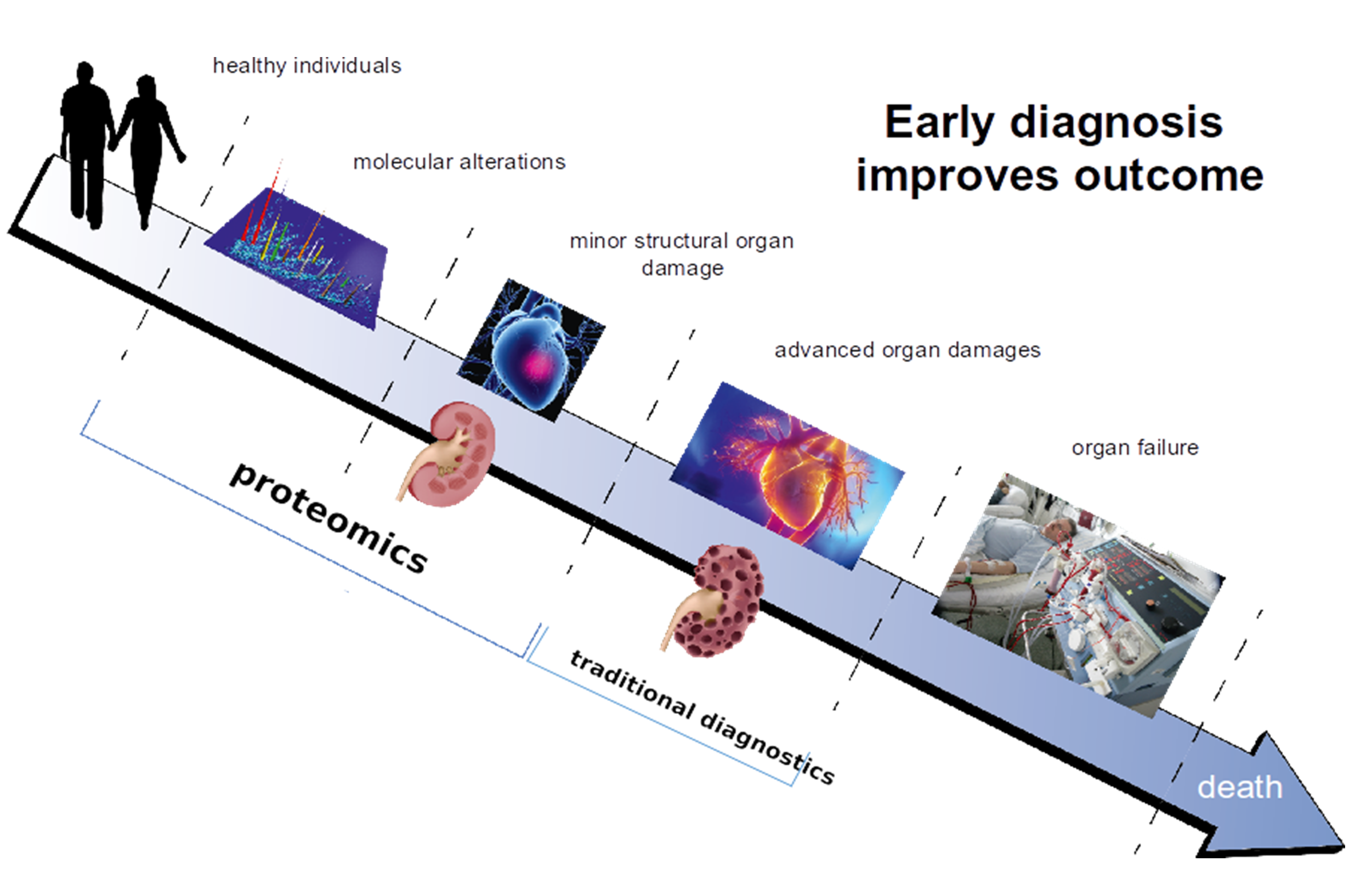
In an effort to encourage the application of biomarkers in CKD, the US Food and Drug administration (FDA) issued a ‘Letter-of-Support’ (https://www.fda.gov/media/98846/download) for a biomarker panel based on 273 urinary peptides that allows early detection of diabetic nephropathy and prognosis of progression. This biomarker panel, called CKD273, has been developed as a result of a multi-center European effort, and demonstrated validity in several recent studies, reviewed in e.g. [1;2].
FDA acknowledges that CKD is a major health problem, especially in type 2 diabetes, and states that risk assessment based on available clinical parameters is insufficient, particularly in patients with early stages of disease. The proposed CKD273 biomarker panel enriches clinical trials of early stage diabetic kidney disease with patients who are more likely to progress. The CKD273 biomarkers originate among others from collagen and alpha-1-antitrypsin, proteins which are linked to fibrosis and inflammation, processes that are thought to be of high relevance in CKD progression.
The FDA as the leading regulatory agency is actively supporting efforts to improve the situation of CKD patients especially at early stages of the disease. Biomarkers will likely play a significant role in addressing especially chronic disease, ideally at a point in time where the damage to the organ is still reversible.
Issuing of this letter was sparked by data recently published in NDT: in a study encompassing 2672 patients, this biomarker performed significantly better than albuminuria in detecting progressors in early stage CKD [3].
CKD273 is already implemented in the PRIORITY study (www.eu-priority.org), a large multicentre RCT aiming at targeted intervention in very early stages of diabetic nephropathy with spironolactone [4].
REFERENCES
1. Critselis E, Lambers HH. Utility of the CKD273 peptide classifier in predicting chronic kidney disease progression. Nephrol Dial Transplant 2016; 31: 249-254
2. Jankowski J, Schanstra JP, Mischak H. Body fluid peptide and protein signatures in diabetic kidney diseases. Nephrol Dial Transplant 2015; 30 Suppl 4: iv43-iv53
3. Pontillo C, Jacobs L, Staessen JA et al. A Urinary proteome-based Classifier for the early Detection of Decline in Glomerular Filtration. Nephrol Dial Transplant 2016; in press:
4. Siwy J, Schanstra JP, Argiles A et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant 2014; 29: 1563-1570