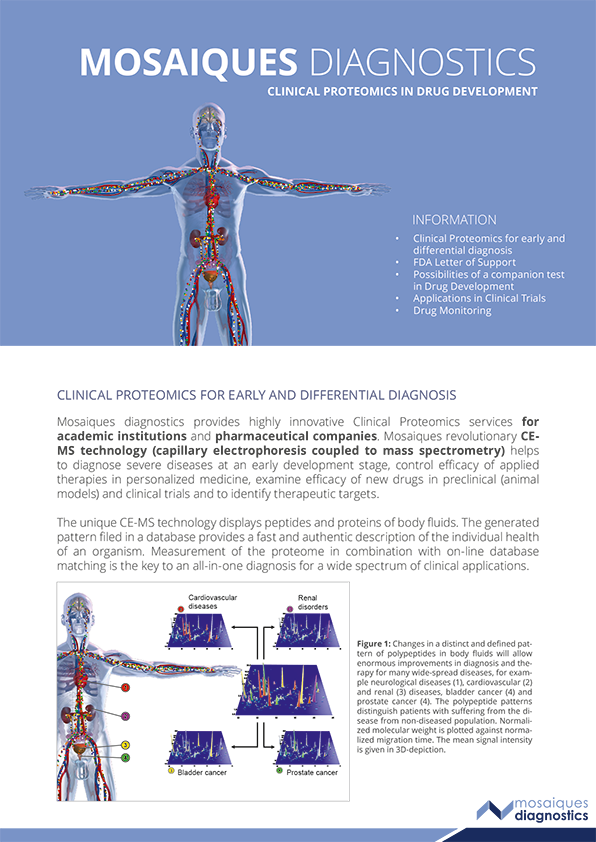
APPLICATIONS
The essential and defining feature of a clinical proteomics study is to obtain robust results that lead to improvement of the current clinical situation. Such improvements may be increased sensitivity and/or specificity in diagnosis, less invasive and/or risk-associated procedures for diagnosis or therapy evaluation, or reduced costs, to name just a few. The results must be clinically relevant and translatable into clinical practice.
A clear understanding of the application of the proteomic test in a clinical setting is needed, in terms of which patients may benefit and what impact the test may have on the outcome of the disease. Such demand requires a clear definition and validation of the assay (technological platform in combination with the biomarkers) to be used for clinical application. Certain platforms (e.g. CE-MS, MALDITOF) may be utilised for discovery, validation, and potentially subsequent clinical application, omitting the need for subsequent translation to another assay system.
The CE-MS technology permits the definition of surrogate markers and endpoints in (pre)clinical trials, allowing evaluation of therapeutic strategies and new drugs on a small number of patients or even animal models. Mosaiques diagnostics has successfully implemented its technology in clinical trials in conjunction with international leading pharmaceutical companies such as Roche Pharma and Bayer Healthcare. Upcoming research projects include, among others, assessment of drug toxicity.
Learn more about the possibilities by implementing our CE-MS technology in clinical development programs and download our latest flyer for pharmaceutical professionals.