Diagnostics
Diagnostics
We at Mosaiques Diagnostics specialise in the field of clinical proteomics; a more targeted approach focusing on identifying biomarkers (e.g. proteins, peptides) to improve current medical practices. As proteins regulate all biological processes, exploring the rich pool of information provided by proteins enable to diagnose chronic diseases with high accuracy and even at an early stage.
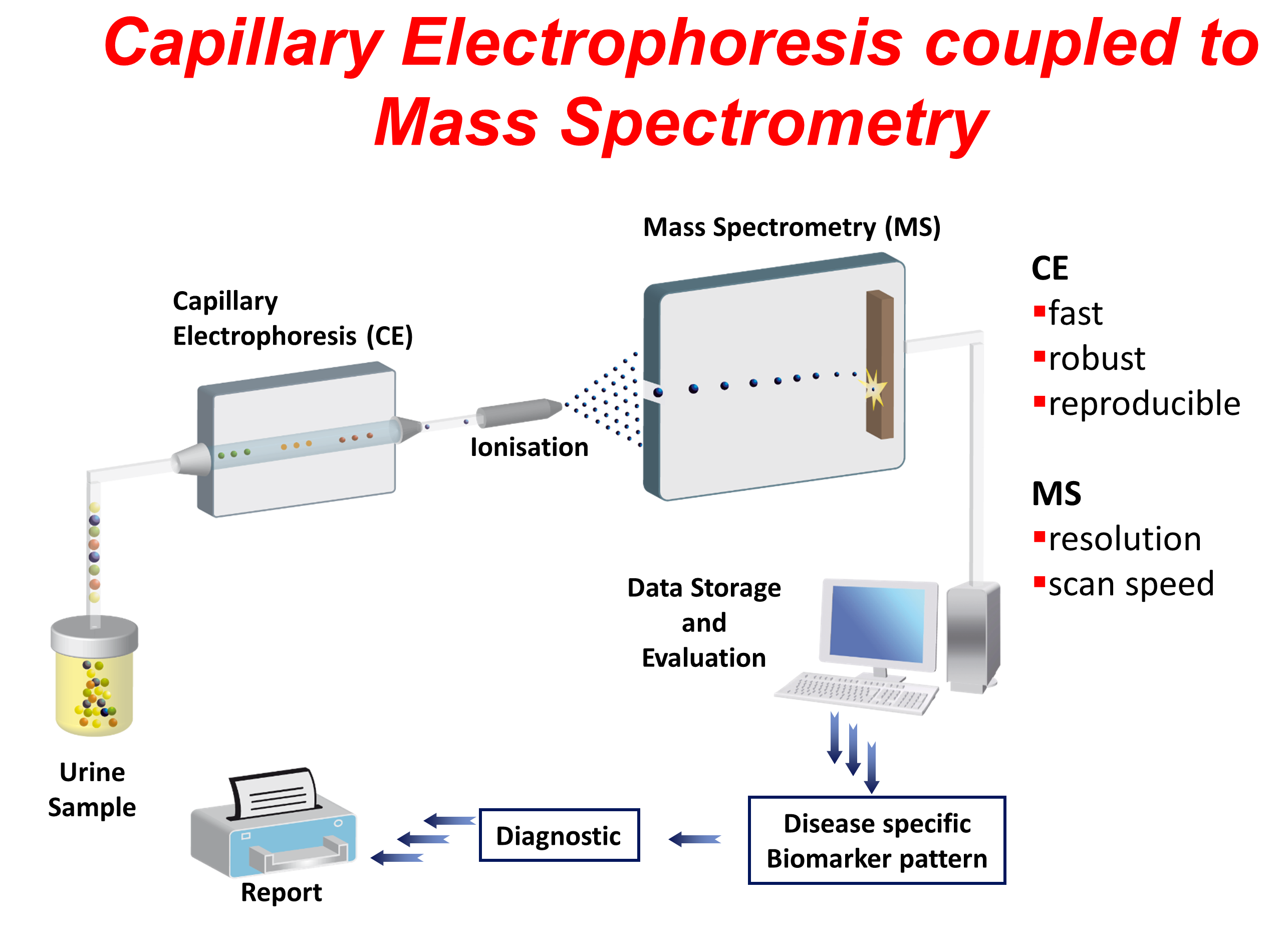
The platform used is called capillary electrophoresis coupled to mass spectrometry (CE-MS) and was invented by Prof. Harald Mischak, the CEO of the company. CE-MS enables reproducible, robust and operator-independent high-resolution detection of up to 6,000 peptides (≥0.8 and ≤18 kDa) in one urine sample1,2. The robustness of the platform in terms of high precision (including inter-laboratory precision), reproducibility and stability were demonstrated in several applications1,2.
Using proprietary tools and algorithms, biomarker (polypeptide) signatures also called classifier can be developed for the diagnosis of pathological conditions. To date, several classifiers have already been developed covering different areas. For more information about commercially available classifiers, please visit this link (https://www.protexam.com/en-gb).
The method was found acceptable by the regulatory agencies European Medicines Agency (EMA) and US Food and Drug Administration (FDA) and has received a “Letter of Support from the FDA (as one of only two European entities to date) encouraging the use of CE-MS-based CKD273 test in the management of chronic kidney disease (CKD)3.
Currently, the following tests are available:
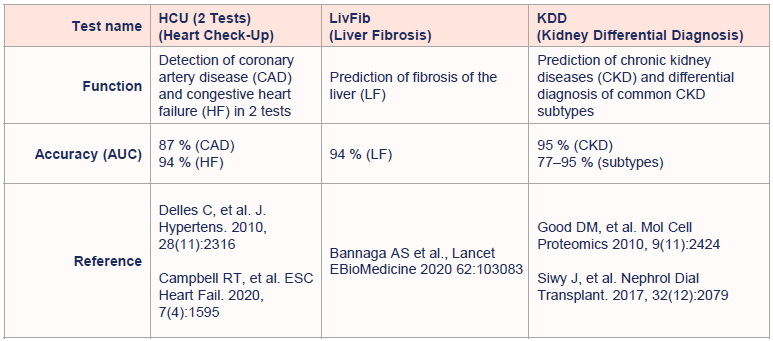
Registered in-vitro diagnostic (IVD) tests for chronic diseases
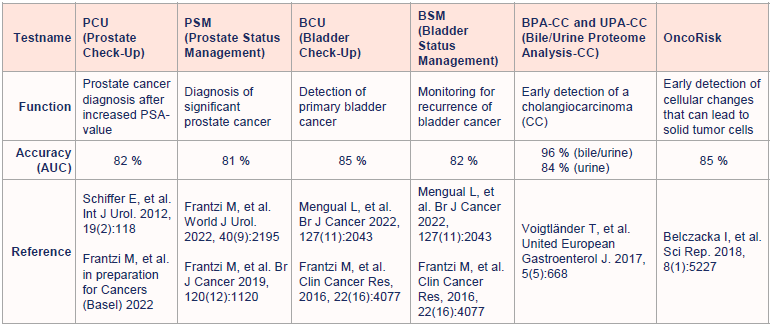
Registered in-vitro diagnostic (IVD) tests for tumor diagnostic
References:
- Haubitz M et al. Mol Cell Proteomics. 2009, 8:2296-2307.
- Mischak H et al. Clin Biochem 2013, 46(6):432-443.
- URL: https://www.fda.gov/media/99837/download
- Mavrogeorgis E et al. Molecules 2021, 26(23):7260.