Animal Models (rat or mice)
We have established and validated protocols for the peptidomic analysis of rodent urine using CE coupled to MS (CE-MS). Our approach provides novel insights into body fluids of animal models, such as rat or mice. Together with peptide identification, the technology is an excellent, complimentary, and non-invasive tool to analyze toxicological or other (patho)physiological effects of pharmaceutical compounds in animal models.
The development and validation of this platform for rodent urine profiling and biomarker definition will open up new vistas in preclinical research, especially in the definition of therapeutic targets, assessment of drug efficacy, and also safety issues (toxicological studies of drugs or toxins). 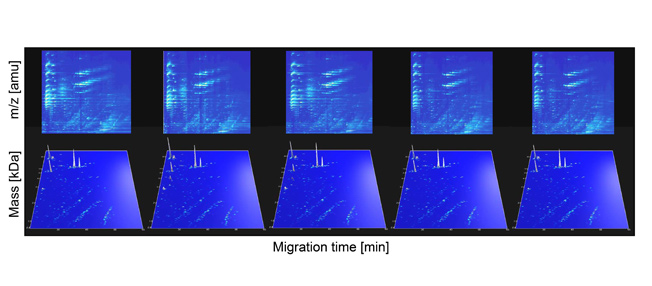
Figure 1: Reproducibility of urinary rat polypeptide evaluation. Examples of electropherograms obtained for 4 (of 19) consecutive measurements of a single urine sample. The m/z values of the 2-D raw data plots (upper panel) and the molecular weight (logarithmic scale) of the deconvoluted 3-D plots (lower panel) on the y-axis are plotted against CEmigration time on the x-axis. The arrangement of the analyzed peptides in distinct lines is obvious, and can be taken as a result of the number of positive charges at pH 2. The white arrow indicates 18.7-kDa signals probably representing major urine protein.
REFERENCES
- Frommberger M et al. Proteomics Clin Appl. 2007; 1(7): 650-60.
- Mischak H et al. Proteomics Clin Appl. 2009; 3(9): 1062-71.
- von zur Muhlen C et al. Mol Cell Proteomics. 2012; 11(7): M111.013847.
- Rouse R et al. PLoS One. 2012; 7(4): e34606.
- Siwy J et al. PLoS One. 2012; 7(12): e51334.
- Dissard R et al. PLoS One. 2013; 8(10): e76703.
- Klein J et al. Kidney Int. 2016; 90(5): 1045-1055.
- Martín-Pozuelo G et al. Int J Mol Sci. 2016; 17(11): 1789.
- Nkuipou-Kenfack E et al. PLoS One. 2017; 12(2): e0166875.
- Liu Y et al. Sci Rep. 2018; 8(1): 5584.
- Mokou M et al. EBioMedicine. 2019; 41: 91-104.



